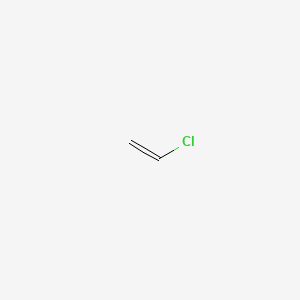
Vinyl chloride will react with bromine water and it will become decolorised.
Distinguish between allyl chloride and vinyl chloride.
In easy words we can call allyl group a merger of methylene bridge and vinyl group as ch 2 methylene bridge is attached with ch ch 2 vinyl group and the rest of the molecule to form allyl group.
The allylic carbon atom is more reactive than normal.
The key difference between these two structural components is the number of carbon and hydrogen atoms.
You have joined no matter what your level.
Ch2 chcl br2 ch2br chclbr ch3ch2cl br2 no reaction i hope this helps.
In vinyl chloride cl is linked with sp2 hybridized carbon whereas in allyl chloride cl is linked with the sp3 hybridized carbon as sp2 hybridized carbon is more electronegative then sp3 hybridized cl can more easily attract the bonded electrons from sp3 carbon and therefore is more reactive.
You can score higher.
Allyl chloride is converted into epichlorohydrin and is utilized for the.
Allyl groups have three carbon atoms and five hydrogen atoms.
Ethyl chloride and vinyl chloride will be distinguished by bromine water.
X well begun is half done.
Key difference allyl vs vinyl both allyl and vinyl groups have slightly similar structures with a small variation.
Ask for details.
The key difference between allyl chloride and vinyl chloride is that ally chloride contains its chlorine atom bonded to the carbon atom that is adjacent to the double bond whereas vinyl chloride contains its chlorine atom bonded to one of the two carbon atoms in the double bond.
Allyl indicates a functional group with structural formula h 2 c ch ch 2 r where r is the rest of the molecule it consists of methylene bridge ch 2 in between the vinyl group ch ch 2 and the rest of the molecule therefore allyl group contains sp 2 hybridized vinyl carbon atoms and sp 3 hybridized allyl carbon atom.
Allylc compounds are diverse and are used in many industries example.
Follow report by kherachohan4870 09 02 2019 log in to add a comment.
Allyl chloride has a ch2 group which takes the part in the saturation process and its hydrogens takes part in the hyperconjugation process.
Ethyl chloride will not decolourise bromine water.
Check your inbox for more details.
How will you distinguish between allyl chloride and vinyl chloride.